Different Types Of Lithium-Ion Batteries, Working, Applications
Hello guys, welcome back to my blog. In this article, I will discuss the different types of lithium-ion batteries, their working, and the applications of different types of lithium-ion batteries.
If you have any doubts related to electrical, electronics, and computer science, then ask questions. You can also catch me on Instagram – CS Electrical & Electronics And Chetan Shidling.
Also, read:
- 1st Oder RC Circuit And 2nd Order RC Equivalent Circuit SoC Estimation
- What Is C Rate In Battery, Why It Is Important, C Rate Calculation
- A Definitive Learner’s Guide For Electric Vehicle Technology Enthusiasts
Different Types Of Lithium-Ion Batteries
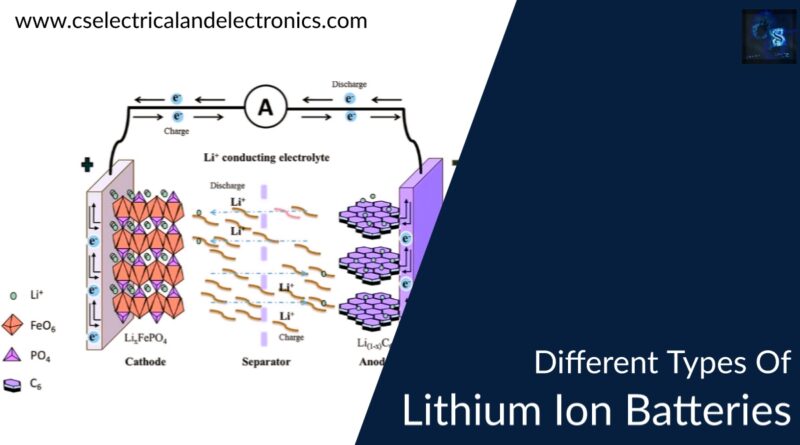
The lithium-Ion battery is a rechargeable battery that is composed of lithium elements in the positive electrode and graphite in the negative electrode. The main working of the lithium-ion battery is the lithium ions move from the positive electrode to the negative electrode during discharging of the battery and while charging the lithium ions will move back from negative electrode to positive electrode.
The lithium ion battery was invented in the year 1958 by Japanese chemist Akira Yoshino as a prototype model later the battery was commercially developed by the Sony company in the year 1991. This battery has an advantage such as high energy density, no memory effect on cells and lithium-ion has low self-discharge property compared to the other electrical batteries. The lithium-ion battery works on the main principle of electrochemical effect.
Construction
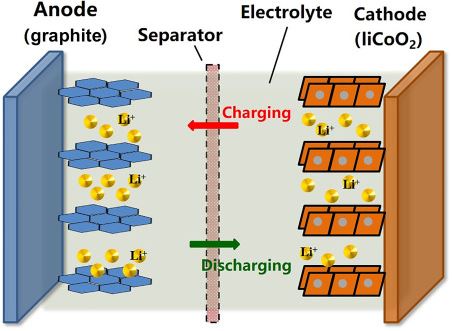
The lithium-ion battery mainly consists of the positive electrode which is popularly known as Anode and a negative electrode which is popularly known as cathode and separator, electrolyte and two current terminals named as positive and negative collectors. The separator is used to separate the electrodes and electrolyte is a solution used for the electrochemistry process. The two current collectors are the outlets of the battery where the consumer can connect the respective load.
Working
The working of the lithium-ion battery is mainly divided into two processes such as Charging and Discharging. During Charging the lithium ions will move from the positive electrode to the negative electrode and the current will be delivered at the outlets of the battery i.e., collectors. During Discharging the lithium ions will move from the negative graphite electrode to the positive lithium electrode where no current will be passed through the current outlets.
The chemical reaction of the lithium ion battery is given below: {LiC6 + CoO2 <=> C6 + LiCoO2}
The above chemical equation from right to left will give you the charging condition and from left to right it is given as the discharging condition. This is the main work involved in the lithium-ion batteries.
There are mainly six types of lithium-ion batteries available in the global market:
01. Lithium Cobalt Oxide Battery
Lithium cobalt oxide batteries mainly consist of three parts anode, cathode and electrolyte. The cathode of lithium cobalt oxide battery is made up of lithium and cobalt elements and the anode is made up of graphite. The electrolyte of the battery is made up of lithium salts. The main work involved in the lithium cobalt oxide battery is the same as the lithium-ion battery. During discharging state the lithium oxide elements made up of cobalt will flow from anode to cathode.
During charging state the lithium oxide elements made up of cobalt will flow from the cathode to the anode. The electrolyte is a salt bridge that is used to make the electrolysis process a smoother way without any changes in the ions exchange between electrodes. This battery is mainly used in portable electronic gadgets such as cell phones, laptops and digital cameras as its energy density is comparatively less and self-discharge property is less. The battery is comparatively smaller in size so the market value of the battery is around 280 US dollars.
02. Lithium Manganese Dioxide Battery
Lithium Manganese dioxide batteries mainly consist of three parts anode, cathode and electrolyte. The cathode of lithium manganese dioxide battery is made up of lithium dioxide elements made up of Manganese and anode is made up of graphite. The electrolyte of the battery is made up of lithium salts. During discharging state the lithium oxide elements made up of Manganese will be flow from anode to cathode. During charging state the lithium dioxide elements made up of Manganese will flow from the cathode to the anode.
The electrolyte is a salt bridge that is used to make the electrolysis process the smoother way without any changes in the ions exchange between electrodes. This battery is mainly used in the microcontrollers and microcomputers power supply and it is mainly used for automatic cameras. The battery is comparatively smaller in size so the market value of the battery is around 3-10 US dollars.
03. Lithium Iron Phosphate Battery
The Lithium Iron Phosphate battery works on the main principle of electrochemical effect. The cathode is made up of a combination of lithium, iron and phosphate but the anode is made up of graphite. The major application of the LFP battery are it is used in passenger cars, logistic vehicles and low speed electric vehicles. The market price of the battery is approximately around 500 US dollars.
04. Lithium Nickel Cobalt Aluminium Oxide Battery
Lithium Nickel Cobalt aluminium oxide battery consists of a cathode made up of Nickel, cobalt and aluminium (NCL) and the negative electrode is made up of carbon graphite. This is one of the trending batteries in the world as it is mostly used in electrical automobiles. This is also used in portable electronic devices. The market price of the lithium NCA batteries starts from the price of 250 US dollars.
05. Lithoium Nickel Manganese Cobalt Oxide Battery
Lithium Nickel Manganese Cobalt oxide battery consists of a cathode made up of Nickel, Manganese and cobalt (NMC) and the negative electrode is made up of carbon graphite. This is one of the trending batteries in the world as it is mostly used in medium speed electrical automobiles. The market price of the lithium NMC batteries starts from the price of 300 US dollars.
06. Lithium Titanate Battery
The lithium titanate battery is the fastest rechargeable battery. The cathode is made up of lithium oxide elements made up of titan element and the anode element is made up of carbon graphite. This is the fastest charging battery and slow rate while discharging, this property made it used in many applications. This batteries can be used for small unit operations and they are also used for electrical bikes as large units. The market price for lithium titanate oxide is Rs 2400/- per unit. This is mostly used in small voltage applications.
This was about “Different Types Of Lithium-Ion Batteries“. I hope this article may help you all a lot. Thank you for reading.
Also, read:
- 10 Tips To Maintain Battery For Long Life, Battery Maintainance
- 10 Tips To Save Electricity Bills, Save Money By Saving Electricity
- 100 (AI) Artificial Intelligence Applications In The Automotive Industry
- 100 + Electrical Engineering Projects For Students, Engineers
- 1000+ Control System Quiz, Top MCQ On Control System
- 1000+ Electrical Machines Quiz, Top MCQs On Electrical Machines
- 1000+ MATLAB Simulink Projects For MTech, Engineering Students
- 50 Tips To Save Electricity At Home, Shop, Industry, Office
Author Profile
- Chetu
- Interest's ~ Engineering | Entrepreneurship | Politics | History | Travelling | Content Writing | Technology | Cooking
Latest entries
 All PostsApril 29, 2024Top 11 Free Courses On Battery For Engineers With Documents
All PostsApril 29, 2024Top 11 Free Courses On Battery For Engineers With Documents All PostsApril 19, 2024What Is Vector CANoe Tool, Why It Is Used In The Automotive Industry
All PostsApril 19, 2024What Is Vector CANoe Tool, Why It Is Used In The Automotive Industry All PostsApril 13, 2024What Is TCM, Transmission Control Module, Working, Purpose,
All PostsApril 13, 2024What Is TCM, Transmission Control Module, Working, Purpose, All PostsApril 12, 2024Top 100 HiL hardware in loop Interview Questions With Answers For Engineers
All PostsApril 12, 2024Top 100 HiL hardware in loop Interview Questions With Answers For Engineers